Virus Tools for Sparse labeling
Virus Tools for Sparse labeling
The mammalian brain, a complex organ, comprises numerous cells (neurons) densely packed and interconnected to form intricate neural circuits responsible for higher brain function. In order to understand how neural circuits develop and function, single-cell analyses are essential that dissect connections among individual cells and the molecular machinery operating within them.. Since neurons have tightly entangled axons and dendrites and are difficult to distinguish, single-cell labeling is challenging.
At present, virus tools have been widely used in neural tracing research, including AAV, SFV, RV, and VSV, which are commonly used for sparse labeling. After these viruses were injected into a specific brain area, the reporter gene was expressed for a period of time, which was followed by optical imaging analysis and FMOST to determine if neurons in that area were sparsely labeled. We can provide packaging services for a variety of virus vectors that are used for sparse labeling.
Table1 Characteristics of different types of viral tools used for sparse highlighting labeling
| Virus type | Labeling type | Characteristics |
| Vesicularstomatitis virus, VSV | Local labeling | Broad host range, fast and high-brightness labeling, and high cytotoxicity |
| Semliki forest virus, SFV | Local labeling | Broad host range, fast and high-brightness labeling, and high cytotoxicity |
| Adeno-associated virus, AAV | Local labeling or retrograde labeling | Various serotypes, low immunogenicity, low cytotoxicity and cell type specific labeling |
| Rabies virus, RV | Retrograde labeling or retrograde trans-monosynaptic labeling | Broad host range, retrograde labeling and cytotoxicity in long-term infections |
| Canine adenovirus, CAV | Retrograde labeling | Low cytotoxicity and achievable cell type-specific labeling |
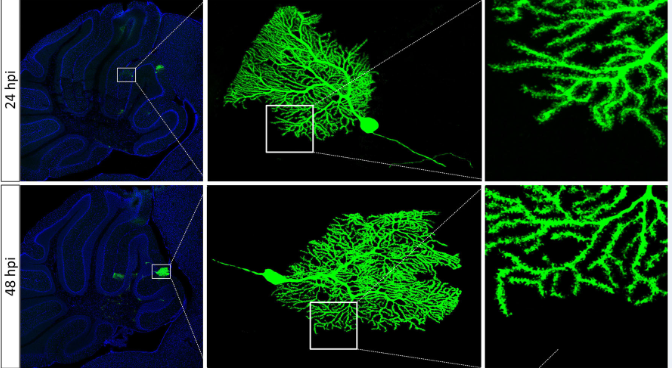
● Sparse labeling based on the single virus system.
As a result, VSV, SFV, RV, and CAV have high infection efficiency. By reducing the viral titer, rapid, sparse and bright cell labeling of local neurons can be achieved by expressing high amounts of the fluorescent protein. Fast bright cell labeling is possible with these tools, but they are cytotoxic, so they can be used for sparse dendrite labeling, but not long-term projection.
● Sparse labeling based on TRE leakage combined with tTA/TRE.
Post-division neurons can be labeled with this method, and the labeling can last up to 8 months. However, dividing neurons cannot be labeled, since the vector becomes diluted and fluorescence intensity decreases.
● Sparse labeling based on Cre/FLP recombinase.
With this method, the injection volume of the virus is adjusted so that a certain number of bright neurons appear in the target area between 10 and 50. The cells so labeled are exquisitely shaped that they can be traced to some extent.
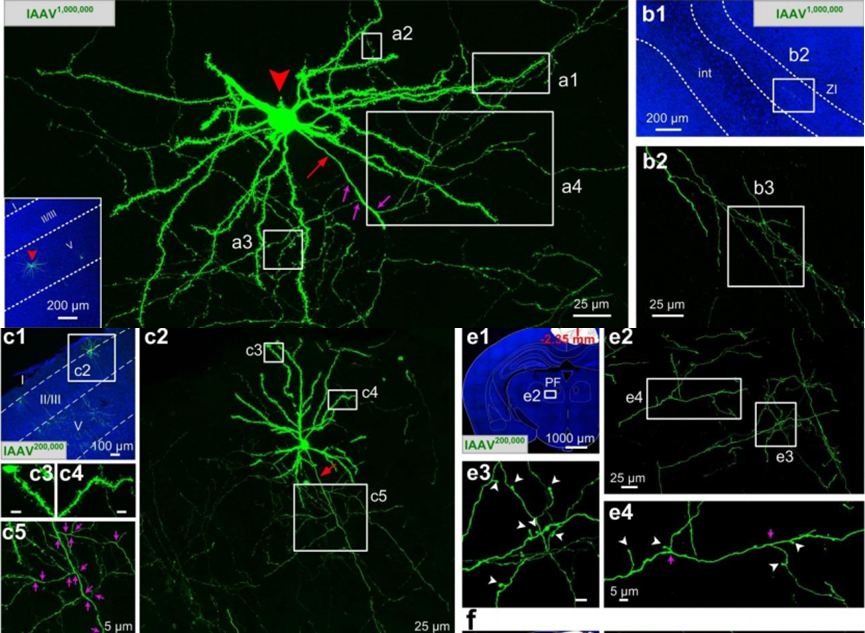
● Sparse labeling based on recombinase system-dependent co-packaging strategy
By co-packaging different rAAVs (including Cre-lox/Flp-FRT), mutual inhibition is improved and compatibility is enhanced. As a result, we were able to capture massive morphological details of individual neurons with a 5 to 10 fold greater sensitivity than a mixture of independently packaged rAAVs.
Our supplier has extensive experience in sparse labeling and could offer optimum experimental conditions according to your requirements. If you have any needs, just email us at [email protected].
References
1. Elife. 2016 Jan 20;5:e10566. doi: 10.7554/eLife.10566.A platform for brain-wide imaging and reconstruction of individual neurons.
2. Nature. 2012 Dec 13;492(7428):247-51. doi: 10.1038/nature11601.
Nonlinear dendritic integration of sensory and motor input during an active sensing task.
3. Sci Rep.2016 Oct 24;6:35747. doi: 10.1038/srep35747.
Supernova: A Versatile Vector System for Single-Cell Labeling and Gene Function Studies in vivo.
4. Sci Rep. 2017 Mar 8;7:43915. doi: 10.1038/srep43915.
Genetically-directed Sparse Neuronal Labeling in BAC Transgenic Mice through Mononucleotide Repeat Frameshift.
5. Brain Struct Funct. 2015;220(3):1369-79. doi: 10.1007/s00429-014-0730-z.
An anterograde rabies virus vector for high-resolution large-scale reconstruction of 3D neuron morphology.
6. J Comp Neurol. 2009 Oct 20;516(6):456-81. doi: 10.1002/cne.22131.
Viral strategies for studying the brain, including a replication-restricted self-amplifying delta-G vesicular stomatis virus that rapidly expresses transgenes in brain and can generate a multicolor golgi-like expression.
7. bioRxiv preprint first posted online Jul. 17, 2019; doi: http://dx.doi.org/10.1101/705772.
Recombinase system-dependent copackaging strategy for highly efficient neurocircuit tracing.
 Loading ....
Loading ....
