FAQ DENARASE What can DENARASE® be used for?DENARASE
® specifically hydrolyses the phosphodiester bonds between nucleotides, leaving smaller fragments of approximately 3-5 base pairs. The enzyme is active on all forms of nucleic acids, including single-stranded, double-stranded, linear, circular or supercoiled. DENARASE
®-mediated removal of unwanted residual plasmid and host cell-derived nucleic acids can significantly improve the purification and yield of viral vectors and proteins. It is therefore widely used in research, process development and production of biologicals such as viral vectors and vaccines.
How should we add DENARASE® to our process?There are several publications on how to use endonucleases derived from
Serratia marcescens, such as DENARASE
®. We recommend starting with concentrations between 10 and 60 U/mL. Several parameters such as time, concentration and temperature are highly dependent on your process conditions and should be evaluated accordingly.
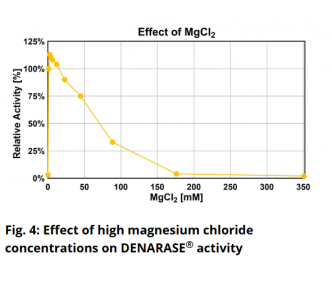
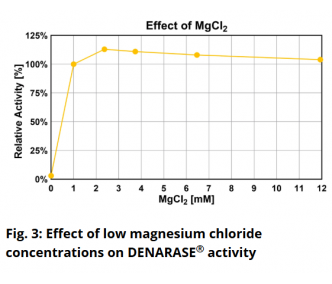
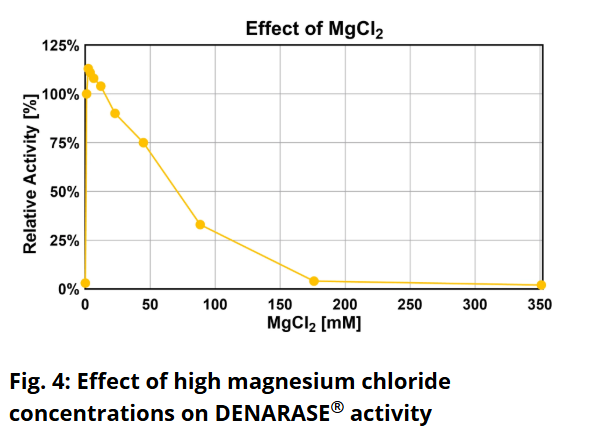
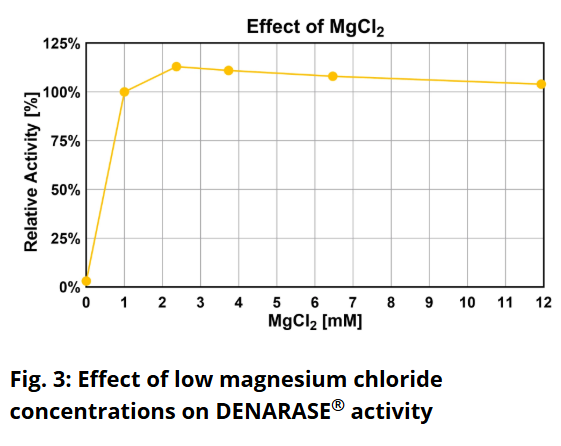
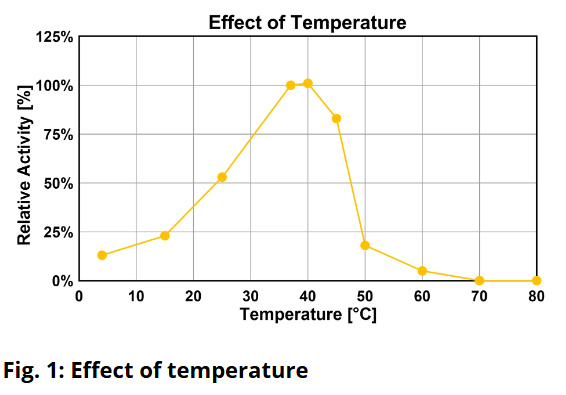
Is DENARASE® active at higher salt concentrations?The optimum salt concentration for DENARASE
® is < 20 mM for monovalent ions. As shown in Figure 7 of the DENARASE
® Product Information Sheet (
DENARASE PIS), enzyme activity decreases with increasing salt concentration (KCl & NaCl). We therefore suggest that the concentration of monovalent cations such as Na
+ and K
+ should be kept below 200 mM for optimal DENARASE
® activity.
Is there any risk for endotoxins? Endotoxins are lipopolysaccharide-protein (LPS) complexes that are part of the outer membrane and are released during the lysis of Gram-negative bacteria such as
E. coli. They are normally detected via the Limulus Amebocyte Lysate (LAL) test. The production host of DENARASE
® is a Gram-positive Bacillus strain, classified as GRAS (Generally Recognized As Safe) and not releasing LPS. The risk of endotoxins originating from the production strain is therefore very low. The general risk of endotoxin contamination, e.g. coming from process raw materials, devices, environment, etc., is carefully assessed, resulting in consistently low endotoxin levels of < 0.25 EU/kU on the specification (as evaluated by LAL testing; see page 4
DENARASE PIS).
How can DENARASE® be removed from my process?A variety of methods are available to remove DENARASE
® from target products. Depending on the characteristics of the process, anion exchange, cation exchange, hydrophobic interaction, hydroxyapatite or size exclusion can be applied. Alteatively, filtration techniques can be used for specific process solutions. Depending on the target molecule, the type of chromatography or filtration media must be tested for the specific case.
Are there different quality grades for DENARASE®?DENARASE is available in two quality grades: DENARASE
® for research and development (R&D) use and DENARASE
® for manufacturing under GMP. GMP-grade DENARASE
® is manufactured under EU GMP conditions and provided with dedicated regulatory support for market approval e.g. via an own US FDA Drug Master File. R&D-grade DENARASE
® is produced in conformity with ISO 9001 standard with less strict requirements regarding documentation, storage and distribution. The R&D-grade is suitable for R&D stages, when fast and easy access to raw materials is key. From a technical performance perspective, both quality grades are equal and the specification parameters are the same.
How can we detect residual DENARASE® in our process solution?DENARASE
® can be detected using commercially available
Serratia marcescens endonuclease ELISA kits. For the quantitative analysis of residual
S. marcescens endonucleases - including Benzonase® Nuclease (registered trademark of Merck KGaA) - in your process samples, we offer our DENARASE
® ELISA Kit. Based on highly specific monoclonal antibodies, our kit provides unmatched reproducibility and the lowest endonuclease detection and quantification limits compared to similar products.

 Loading ....
Loading ....