Studies have found that when a single cardiac progenitor differentiates into a anterior artery, angiogenesis fate will s

Studies have found that when a single cardiac progenitor differentiates into a anterior artery, angiogenesis fate will shift

Copyright © iCell Bioscience Inc, Shanghai 2018-2019
A key feature of cells during organogenesis and regeneration is the transformation of fate and the acquisition of new identities. However, little is known about the underlying mechanisms of this transformation. The vascular system differentiates into arteries and veins during embryogenesis through an antagonistic transcriptional program, providing a biological model for understanding this transformation process. These antagonistic transcriptional programs include a Notch signal for maintaining arteries and COUP-TF (also known as NR2F2) for maintaining veins.
During embryogenesis, a portion of the coronary arteries in the heart are derived from a vein called the sinus (SV). Although the vein can be a source of new arteries during development, the timing and requirements for this venous-arterial transition are unclear.
Now, in a new study, a team of scientists from cardiovascular medicine and bioengineering developed a statistical test method for single-cell RNA sequencing (scRNA-seq) data in mouse genetics. Cell subpopulations are classified and identified as a population of cells that direct developmental transition during cardiac formation.
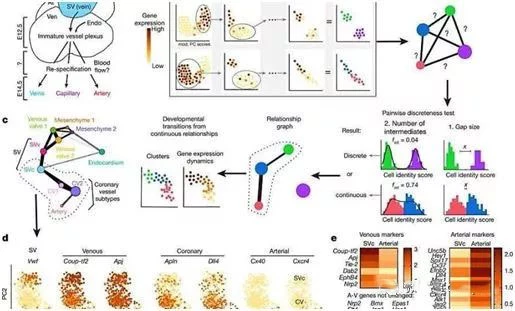
This study combines statistical analysis, computational analysis, and biological analysis of venous sinus to demonstrate that venous cells gradually transform their fate from venous cells to arterial cells, and then some cells cross the transcription threshold to enter The state of the anterior artery. Understanding how cell fate changes during development and related inhibition signals will help deepen understanding of tissue growth and organogenesis, thereby improving tissue engineering strategies in regenerative medicine.
In this new study, iterative robust principal component analysis (rPCA) was used, followed by pairwise discrete tests to identify anterior arterial endothelial cell subtypes such as venous sinus coronary artery progenitor cells (SVc) and sinus valve progenitor cells ( SVv) and classify them. These results were confirmed by computational simulations and presented on a graph to identify
developmental changes in endothelial cell subtypes in the ApjCreER transgenic mouse strain. Cell fate shifts can be studied at high resolution by observing changes in gene expression in successive cell populations.
These researchers then removed the lineage-labeled endothelial cells from the mouse embryonic heart on day 12.5 of the embryo for further analysis. Embryonic cells show a shift to arterial fate before the blood begins to flow, so these cells are called anterior arterial cells.
As a latent embryo-specific gap junction protein, connexin (CX40) appeared early in E12.5, suggesting that anterior arterial cells function as cardiac progenitors in the formation of most mature coronary arteries. Further structural changes that occur after blood begins to flow during heart formation are thought to be caused by shear stress.
When a venous-arterial developmental transition is projected onto a linear continuum, the identity of a single venous cell is gradually lost as the cell's forward arterial fate changes. These results indicate that anterior arterial specialization occurs after reaching a threshold for venous identity loss and increased arterial identity; initially, the loss of venous identity is progressive, followed by an increase in arterial identity.
To determine that anterior artery specialization is necessary for arterial formation, these researchers performed a loss of function study by blocking this process in the laboratory. This can be achieved by blocking COUP-TF2, a regulator that normally induces venous fate (while blocking arterial fate). Induction of expression of COUP-TF2 prior to the onset of pre-arterialization prevented the formation of coronary arteries in the venous cells, indicating that the fate of the anterior artery cells could not be obtained.
These data confirm that when a single cardiac progenitor reaches a threshold for differentiation into anterior arterial cells, fate transitions during angiogenesis gradually occur. The anterior artery cells eventually form a mature coronary artery. Basic scientific research conducted in this way helps to gain insight into the mechanisms of cell fate transformation and provide potential therapeutic applications. Future experiments can study the differentiation of cardiac arteries during angiogenesis, which can help develop regenerative therapies.
 Loading ....
Loading ....
